Domestic Cleaners
Are you looking for more than just a cleaner? I am DBS checked self employed cleaner and have a full driving licence. Your home will be in good hands, well looked after and will sparkle once I have been around and given it that special touch. If you would like your home to be given the ‘Fairy Sparkles’ treatment, please get in touch.
Domestic Cleaning Service
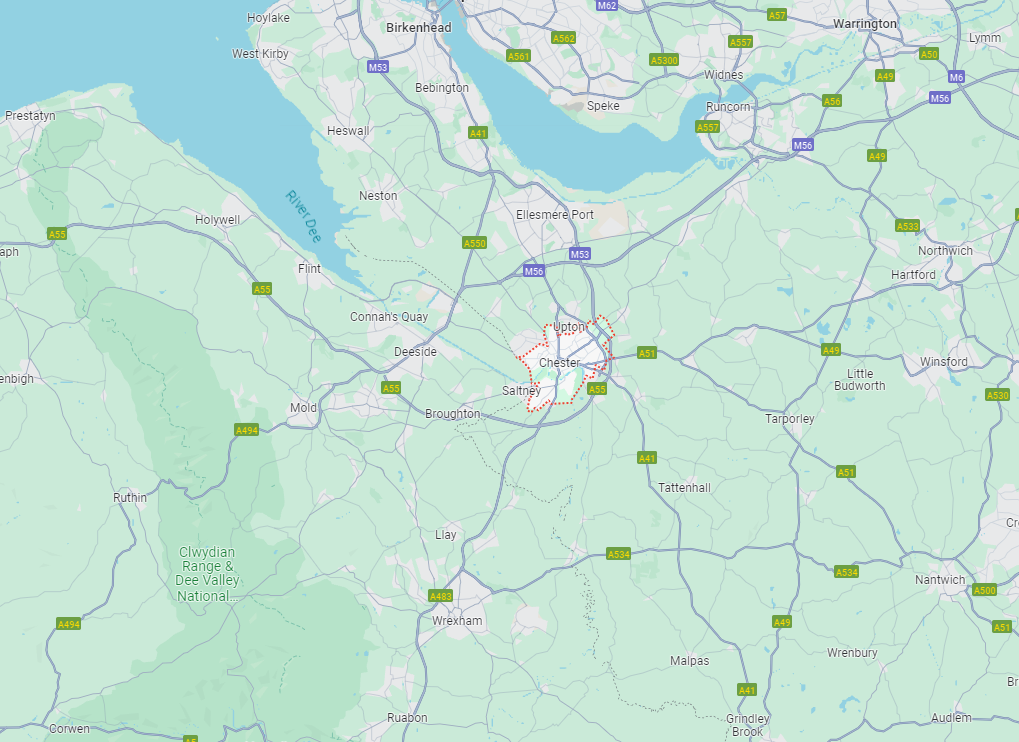
Affordable Cleaning services in Chester, Wrexham, Frodsham and throughout Cheshire

Full House Cleaning Service
Looking after a full house is my speciality. Keeping everything in order, neat & tidy as well as being clean & smelling lovely.
Your house should feel like a clean home. Regain your sparkle with Fairy Sparkles.

Regular House Cleans
The most popular cleaning service is a regular house clean, this can be either weekly, fortnightly or monthly.
It allows me to keep on top of the little jobs and give you a regular all round sparkling feeling.

Cleaning for Special Occasions
Preparation for a special occasion like Christmas, Easter, Birthday or family gathering.
Have your home sparkling and clean for the big day, don’t forget to get in touch if you want me to clean up afterwards too!

Kitchens Cleaned
Deep clean of worktop surfaces, tops & faces of cupboards, hob, sink & drainer, windows, floor & skirting boards.
Anti-bacterial products used to keep away the germs!
Bathroom Cleaning Service
A deep and fresh clean of everything in your bathroom & en-suite.
A sparkle on your sink, toilet, bath & shower using anti-bacterial & bleach products to keep all the germs away.

Domestic Room Cleans
Hoovering, mopping, dusting & wiping down of surfaces to give your rooms a good clean.
Home offices & games rooms also catered for. Everything in sight will be given the Fairy Sparkles treatment.
Am I one of the best cleaners in the area? Many people think so
Species of germs in the average household - Tel or Message for Cleaning Services


Areas Covered by Fairy Sparkles Cleaning Services
Cleaning Services – (Affordable Self Employed Cleaner)
- Cleaning Services Near Me in Bangor-on-Dee
- Cleaning Services Near Me in Borras
- Cleaning Services Near Me in Buckley
- Cleaning Services Near Me in Caerwys
- Cleaning Services Near Me in Cheshire
- Cleaning Services Near Me in Chester
- Cleaning Services Near Me in Christleton
- Cleaning Services Near Me in Deeside
- Cleaning Services Near Me in Elton
- Cleaning Services Near Me in Ewloe
- Cleaning Services Near Me in Frodsham
- Cleaning Services Near Me in Gresford
- Cleaning Services Near Me in Halkyn
- Cleaning Services Near Me in Hawarden
- Cleaning Services Near Me in Helsby
- Cleaning Services Near Me in Higher Kinnerton
- Cleaning Services Near Me in Hooton
- Cleaning Services Near Me in Kingsley
- Cleaning Services Near Me in Manley
- Cleaning Services Near Me in Marchwiel
- Cleaning Services Near Me in Mollington
- Cleaning Services Near Me in Mouldsworth
- Cleaning Services Near Me in Mynydd Isa
- Cleaning Services Near Me in Nannerch
- Cleaning Services Near Me in New Brighton
- Cleaning Services Near Me in Northop
- Cleaning Services Near Me in Overton
- Cleaning Services Near Me in Penley
- Cleaning Services Near Me in Penyffordd
- Cleaning Services Near Me in Preston Brook
- Cleaning Services Near Me in Rhosddu
- Cleaning Services Near Me in Rhosnesni
- Cleaning Services Near Me in Rossett
- Cleaning Services Near Me in Ruabon
- Cleaning Services Near Me in Runcorn
- Cleaning Services Near Me in Saltney
- Cleaning Services Near Me in Stretton
- Cleaning Services Near Me in Sutton Weaver
- Cleaning Services Near Me in Warrington
- Cleaning Services Near Me in Waverton
- Cleaning Services Near Me in Widnes
- Cleaning Services Near Me in Wrexham
- Cleaning Services Near Me on the Wirral
Let me do your cleaning so you can enjoy free time
